The European Medical Device Nomenclature is an essential document for all MD manufacturers who wish to place their products on the market. The first, essential step is to define the class assigned to the product. This is indeed of great importance. This step will define the regulatory requirements which will need to be met to ensure the device’s compliance. There are four classification levels (I, IIa, IIb and III) ranging from the lowest to the highest risks.
The Medical Device Nomenclature (EMDN), used by manufacturers during the EUDAMED registration process for medical devices, has an alphanumeric structure. It has a seven-level hierarchical tree. It clusters medical devices into three main levels: categories, groups and, the last level, types. These types are available on several levels. The alphanumeric code generated starts with a letter that refers to the category of the device. The next two numbers refer to the group, and the last series of numbers to the type of MD. However, a maximum limit has been set аt 13 characters.
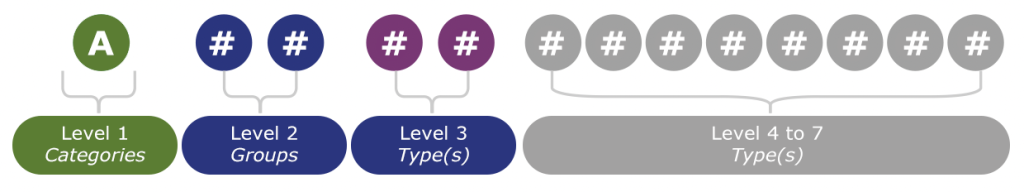
Therefore, manufacturers must thoroughly assess the impact of extended definitions and classification changes in relation to their current and future product portfolios. Requirements differ based on the product classification.
To learn more about the European Medical Device Nomenclature, please view our FAQ below. Do not hesitate to contact us for more information.
How was the European Medical Device Nomenclature (EMDN) created?
In accordance with the criteria and requirements set out by the European Commission and EU regulators through the Medical Device Coordination Group (MDCG), and based on its guidance, the EMDN was created following a communication from the European Commission providing for the use of the Italian Ministry’s “Classificazione Nazionale Dispositivi medici (CND)” as a basis for its future development.
At that time, the CND was already being used in three Member states (Italy, Greece and Portugal) and enabled the registration of multiple European and international manufacturers within the EU. In 2019 and 2020, stakeholders and key experts worked together to conduct consultations and preparatory work on the CND. A first version of EMDN was published on 4 May 2021.
What are the main principles of EMDN?
The EMDN is based on fundamental key principles jointly set out by the European Commission and EU regulators. These principles include but are not limited to:
- Regulator-led: regulators play a key role in managing, validating, updating and advising on the nomenclature.
- Structured: the nomenclature has transparent hierarchies by which terms and codes could be meaningfully clustered into groups and types.
- Predictable: the structure and content remain sufficiently stable to allow various regulatory uses of the nomenclature, in a manner which still allows for the accommodation of technological innovation.
- Transparent: the policies for updates of the nomenclature terms and descriptions are sound and reflect the needs of regulators and the wider healthcare community.
- Inclusive: the periodic reviews are open to all, based on real-world use and demonstrable needs.
- Available: the terms, descriptions and codes are available in full to all users.
- Accessible: no manufacturer or natural/legal person should be subject to a fee or suffer from any discrimination, compared to other operators, in relation to the use of the nomenclature.
- International: internationally recognised at the time of the date of application of the MDR/IVDR.
How I gain access to the EMDN?
The entirety of the EMDN is accessible to all stakeholders, free of charge. It can therefore be used by a non-exhaustive list of stakeholders such as manufacturers, patients, research organisations, doctors, hospitals, pharmacies, etc. The EMDN can be viewed and downloaded in PDF and Excel formats on the European Commission website and on the MDCG documents page of the European Commission.
Please note: The European Commission organised a one-month online consultation on the English version of the EMDN, which was open until 4 June 2021. The purpose of this consultation was to gather feedback from users and the wider healthcare community on any translation errors and/or syntax suggestions. Following the processing of the comments provided, the second version of EMDN will be released in Q3 2021. In addition, new terms and descriptions for medical device software (under Categories J, W and Z) will be rolled-out in the second release.
Themes


















