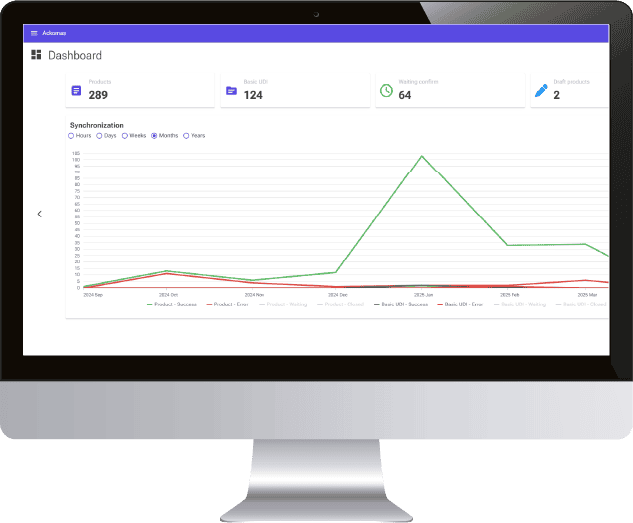
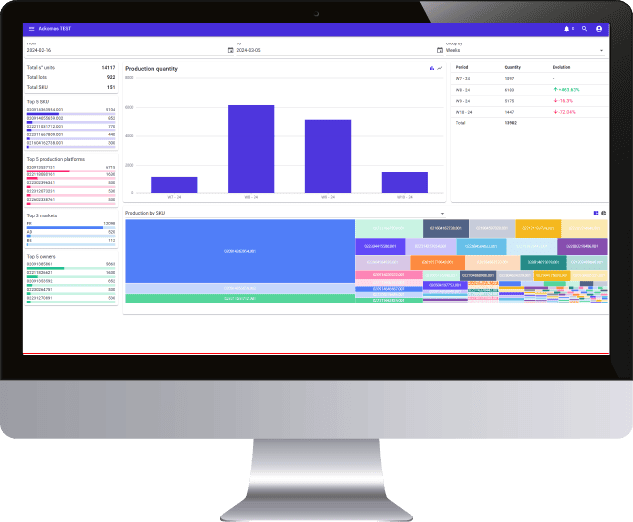
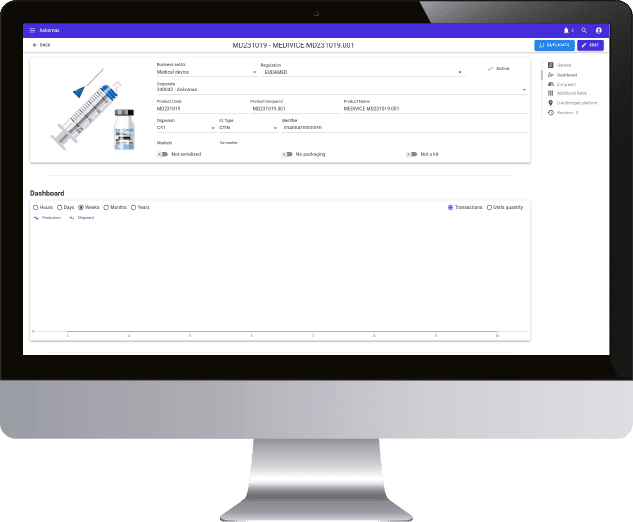
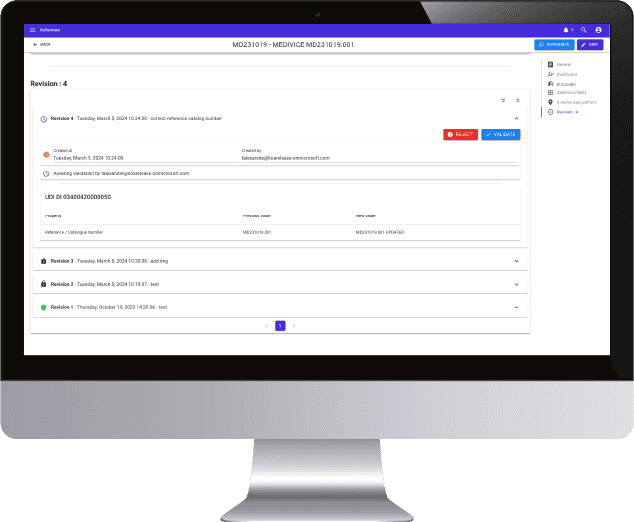
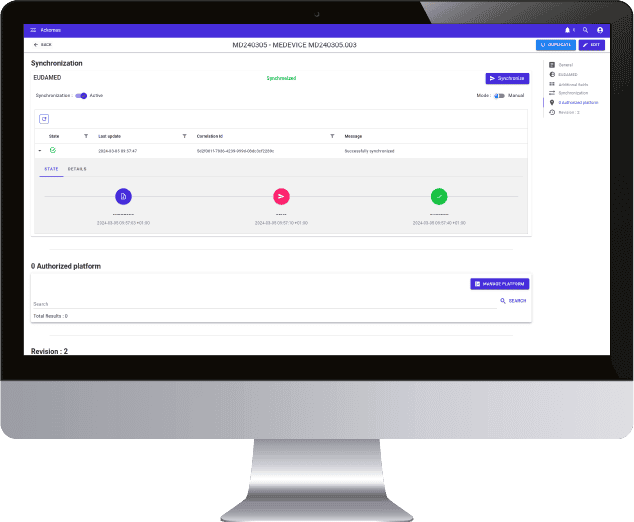
20 years of expertise in M2M connection protocols, real sensitivity to data integrity, daily regulatory and legislative support, all the markers are green to create a real collaboration with Ackomas on the management of master data and Eudamed On Boarding.